Our Phase II Study - RAINBOW
RAndomized, double-blINd, placeBo-controlled, multicenter, 12-week study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of Oral nizubaglustat in patients With GM2 Gangliosidosis or Niemann-Pick disease type C (NPC).
This study aimed to assess the correct doses of nizubaglustat to treat GM2 and NPC diseases by evaluating the clearance of nizubaglustat from the body and the effect of two different doses in a small number of patients with those diseases
Study Design
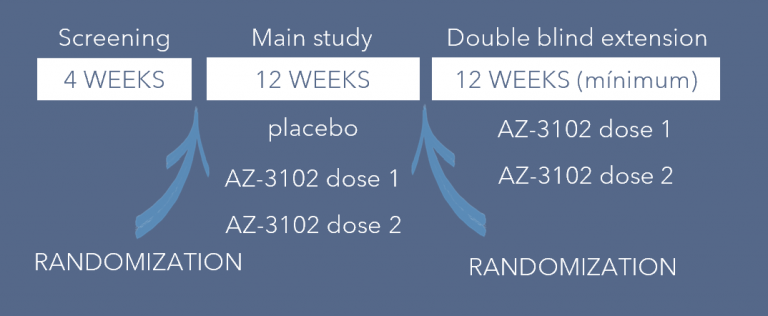
RAINBOW was designed as a short, multinational study, which involved 13 patients older than 12 years with GM2 Gangliosidosis or Niemann-Pick disease type C (NPC). Enrolment was initiated in early 2023, and each participant had a duration study time of three months.
The study is now complete and we are looking forward to analysing the data and presenting the results.