RAINBOW Study
Our Phase II Study - RAINBOW
RAndomized, double-blINd, placeBo-controlled, multicenter, 12-week study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of Oral nizubaglustat in patients With GM2 gangliosidosis or Niemann-Pick type C (NPC) diseases.
This study aims to assess the correct doses of nizubaglustat to treat GM2 and NPC diseases by evaluating the clearance of nizubaglustat from the body and the effect of two different doses in a small number of patients with those diseases.
Study Design
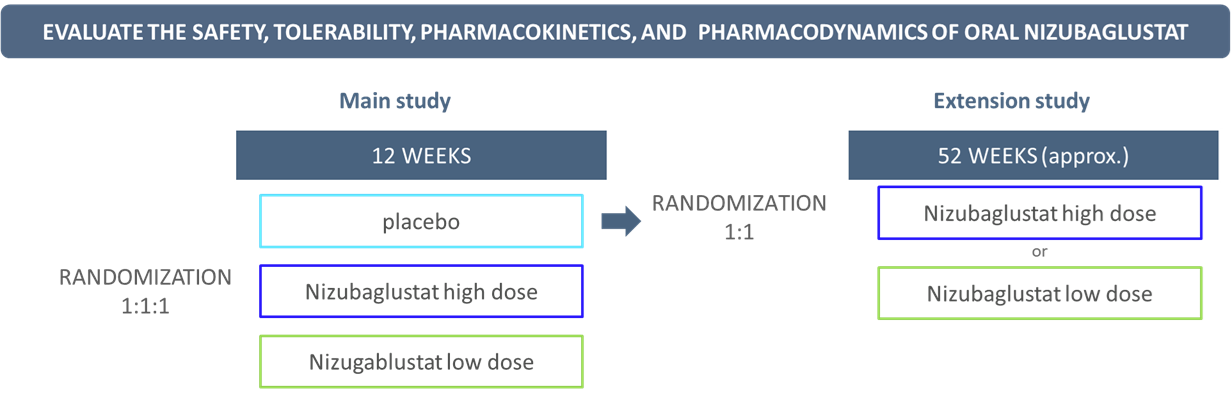
RAINBOW was designed as a short study, with 12 participants (six per disease) older than 12 years with late-infantile or juvenile onsets. The main part of the study lasted 12 weeks and ended in February 2024. Patients have since been move into the extension phase, which is ongoing. All of them are now taking the study drug.
More information?
If you have any questions about this study, please email us at: info@azafaros.com.
